neon neutrons|Neon (Ne) : Manila Neon is the 10th element in the periodic table and has a symbol of Ne and atomic number of 10. It has an atomic weight of 20.1797 and a mass number of 20. Neon has ten protons . [gyobobo] Claire Set v1.0.zipmod: 2021-10-29 07:00 : 7.6M [gyobobo] eva4 v1.0.zipmod: 2023-02-03 19:52 : 33M [gyobobo] Fumina Set v1.2.zipmod: 2021-05-29 13:34 : . [gyobobo]Muta Set.zipmod: 2020-06-04 13:26 : 76K [gyobobo]Natsumi Expansion Set.zipmod: 2020-06-04 13:28 : 5.4M [gyobobo]Natsumi Set.zipmod: 2020-06-04 13:28 .
neon neutrons,Element Neon (Ne), Group 18, Atomic Number 10, p-block, Mass 20.180. Sources, facts, uses, scarcity (SRI), podcasts, alchemical symbols, videos and images. Jump to main content
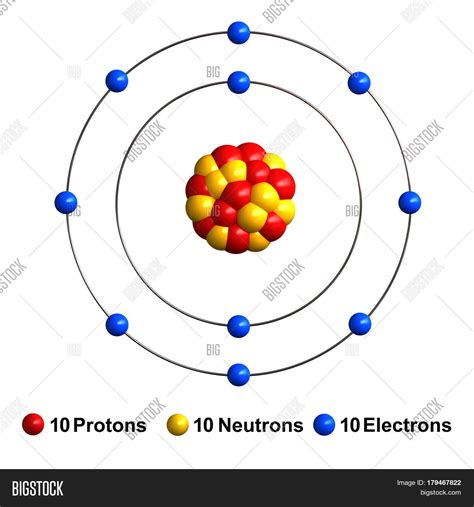
neon neutronsNeon has three stable isotopes: Ne (90.48%), Ne (0.27%) and Ne (9.25%). Ne and Ne are partly primordial and partly nucleogenic (i.e. made by nuclear reactions of other nuclides with neutrons or other particles in the environment) and their variations in natural abundance are well understood. In contrast, Ne (the chief primordial isotope made in stellar nucleosynthesis) is not known to b.Neon is the 10th element in the periodic table and has a symbol of Ne and atomic number of 10. It has an atomic weight of 20.1797 and a mass number of 20. Neon has ten protons .Name: Neon. Symbol: Ne. Atomic Number: 10. Atomic Mass: 20.1797 amu. Melting Point: -248.6 °C (24.549994 K, -415.48 °F) Boiling Point: -246.1 °C (27.049994 K, -410.98 °F) Number of Protons/Electrons: 10. . The abundances of the naturally occurring isotopes of neon. Neon (10 Ne) .

Neon is a noble gas with three stable isotopes: 20 Ne, 21 Ne and 22 Ne. Learn about its discovery, properties, uses and more from this web page. Neon is a noble gas with three stable isotopes: 20 Ne, 21 Ne and 22 Ne. Learn about its discovery, properties, uses and more from this web page.Neon is a chemical element of the periodic table with chemical symbol Ne and atomic number 10 with an atomic weight of 20.1797 u and is classed as noble gas and is part of .
Neon is in the air all around us, but it’s only a small part of air: 54,900 liters of dry air contains 1 liter of neon. Another way of saying this is that air contains 0.00182% neon by volume. We get neon by fractional .

Neon is the second lightest noble gas in the noble gas family. (Helium is the lightest of all the noble gases.) Neon gas is lighter than air so it always goes up in .Neon - Properties, history, name origin, facts, applications, isotopes, electronic configuation, crystal structure, hazards and more; Interactive periodic table of the chemical elements.Notes (related to the columns): 1 - name of the nuclide, isotope. 2 - E: isotope symbol with mass number (superscript; number of nucleons) and Atomic number (subscript; number of protons). 3 - N: number of neutrons. 4 - relative atomic mass of the Neon isotope (isotopic mass including electrons) and the mass of the atomic nucleus in square brackets . In this video we’ll use the Periodic table and a few simple rules to find the protons, electrons, and neutrons for the element Neon (Ne). From the Periodic T.Interesting Facts about Neon. 0.0018 percent of Earth’s atmosphere is neon. Although it is relatively rare on our planet, neon is the fifth most abundant element in the universe. If you could gather all the neon . There are many isotopes of neon and each has a different number of neutrons. Neon always has 10 protons, but the number of neutrons varies with each isotope. The most common are 20 10N e (10 protons, 90.48% abundance), 21 10N e (11 protons, .27% abundance), and 22 10N e (12 protons, 9.25% abundance). Other, .Neon (Ne)Radioactive. No. From the Greek word neos, new. Crystal Structure. Face Centered Cubic. History. Neon was discovered in 1898 by the British chemists Sir William Ramsay and Morris W. Travers in London. It was discovered when Ramsay chilled a sample of air until it became a liquid, then warmed the liquid and captured the gases as they boiled off. 10 Facts About Element No. 10. Each neon atom has 10 protons. There are three stable isotopes of the element, with atoms having 10 neutrons (neon-20), 11 neutrons (neon-21), and 12 neutrons (neon-22). Because it has a stable octet for its outer electron shell, neon atoms have 10 electrons and no net electrical charge.Number of Neutrons: 10: Number of Electrons: 10: Melting Point-248.6° C: Boiling Point-246.1° C: Density: 0.901 grams per cubic centimeter: Normal Phase . Sir William Ramsay and Morris W. Travers: Common Compounds: There are no common compounds using neon. Interesting facts: It is the fourth most abundant element in the universe, but only .
neon neutrons Neon (Ne)How many protons, neutrons, and electrons are in flouride, nitrogen, boron, beryillium, neon, magnesium, aluminum, helium, carbon, silver, gold, plastic, rubber, and barium each. . 90% of the neon atoms contain 10 neutrons. There are a few with 11 neutrons and around 9% with 12. Those with extra neutrons are called isotopes, the nucleii .
neon neutrons|Neon (Ne)
PH0 · Neon Facts
PH1 · Neon Element Facts
PH2 · Neon (Ne)
PH3 · Neon (Ne)
PH4 · Neon
PH5 · Isotopes of neon
PH6 · Chemical Elements.com